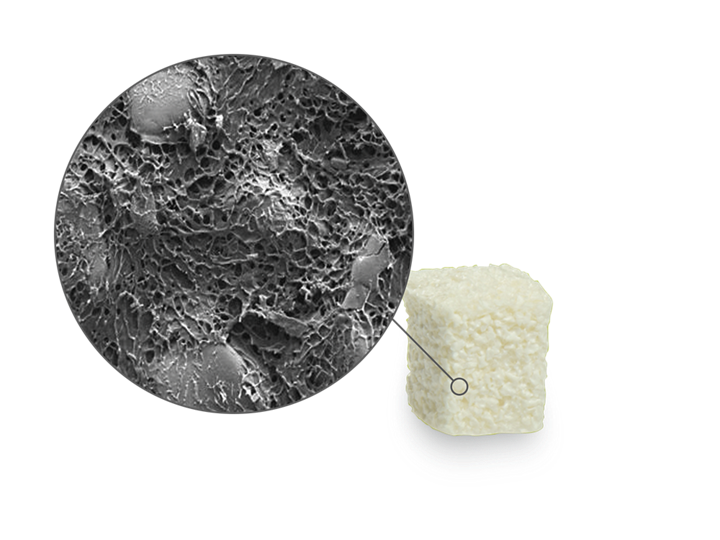
Straumann® XenoFlex is a biomimetric composite material that resembles the native bone in its basic biphasic composition of collagen and xenogenic hydroxyapatite. It has beneficial handling characteristics and the ability to be shaped to match the individual defect situation. Straumann® XenoFlex – an efficient, easy to handle, volume stable solution for the treatment of bone defects.
Straumann® XenoFlex is a biomimetric composite material that resembles the native bone in its basic biphasic composition of collagen and xenogenic hydroxyapatite. It has beneficial handling characteristics and the ability to be shaped to match the individual defect situation. Straumann® XenoFlex – an efficient, easy to handle, volume stable solution for the treatment of bone defects.
FEATURES AND BENEFITS
| Osteoconductivity | The natural structure of Straumann® XenoFlex with interconnected porous granules and purified collagen facilitates the adhesion and invasion of bone forming cells and results in complete integration of the implant due to the ingrowth of cells and blood vessels. |
| Healing environment and volume stability | The collagen portion of Straumann® XenoFlex supports the initial healing environment and binding of the granules to the defect. The collagen creates the environment favorable for bone generation and is decomposed after a certain time (weeks). The granules undergo superficial resorption only. The granules provide excellence space maintenance and predictably integrate into newly formed bone ensuring volume maintenance and a strong long lasting matrix for successful placement of dental implants. |
| Safety | In order to assure maximum safety, organic components are completely removed by solvent and temperature treatment (>500°C) during the manufacturing process of Straumann®Xenoflex. The final sterility of Straumann® XenoFlex is ensured by gamma irradiation. |
| Spongy consistency after hydration | After hydration Straumann® XenoFlex changes to a slightly spongy consistency enabling excellent handling and defect application. The collagen fibers have intrinsic hemostatic properties facilitating the adhesion of proteins and signaling molecules from the blood to the embedded granules to further improve the fast bony integration of Straumann® XenoFlex. |
| Easy handling and application | Straumann® XenoFlex can be easily cut to the needed size and shape in dry and wet condition. The product can be placed into defect in one piece using tweezers shortening operation time. |
FEATURES AND BENEFITS
| Osteoconductivity | The natural structure of Straumann® XenoFlex with interconnected porous granules and purified collagen facilitates the adhesion and invasion of bone forming cells and results in complete integration of the implant due to the ingrowth of cells and blood vessels. |
| Healing environment and volume stability | The collagen portion of Straumann® XenoFlex supports the initial healing environment and binding of the granules to the defect. The collagen creates the environment favorable for bone generation and is decomposed after a certain time (weeks). The granules undergo superficial resorption only. The granules provide excellence space maintenance and predictably integrate into newly formed bone ensuring volume maintenance and a strong long lasting matrix for successful placement of dental implants. |
| Safety | In order to assure maximum safety, organic components are completely removed by solvent and temperature treatment (>500°C) during the manufacturing process of Straumann®Xenoflex. The final sterility of Straumann® XenoFlex is ensured by gamma irradiation. |
| Spongy consistency after hydration | After hydration Straumann® XenoFlex changes to a slightly spongy consistency enabling excellent handling and defect application. The collagen fibers have intrinsic hemostatic properties facilitating the adhesion of proteins and signaling molecules from the blood to the embedded granules to further improve the fast bony integration of Straumann® XenoFlex. |
| Easy handling and application | Straumann® XenoFlex can be easily cut to the needed size and shape in dry and wet condition. The product can be placed into defect in one piece using tweezers shortening operation time. |
PROPERTIES
| Attribute | Description |
| Origin | Bovine cancellous bone particles Porcine collagen type I |
| Composition | 90% Calcium phosphate (100% pure hydroxyapatite, mineral phase) 10% Type I Collagen |
| Structure | Fast binding at defect site due to 10% of porcine collagen, very slow superficial degradation of bovine particles. Long term osseous integration of particles into newly formed bone matrix |
| Thickness | 6–9 months (depending on defect) |
| Degradation time | 12-16 weeks |
| Storage temperature | 2–30°C |
| Shelf life | 3 years (from date of production) |
PROPERTIES
| Attribute | Description |
| Origin | Bovine cancellous bone particles Porcine collagen type I |
| Composition | 90% Calcium phosphate (100% pure hydroxyapatite, mineral phase) 10% Type I Collagen |
| Structure | Fast binding at defect site due to 10% of porcine collagen, very slow superficial degradation of bovine particles. Long term osseous integration of particles into newly formed bone matrix |
| Thickness | 6–9 months (depending on defect) |
| Degradation time | 12-16 weeks |
| Storage temperature | 2–30°C |
| Shelf life | 3 years (from date of production) |
Available in the following sizes
| Code | Dimension L×W×H (mm) |
Product |
| NI-0110-005 | 6 x 6 x 3, 50mg | Straumann® XenoFlex Block |
| NI-0110-010 | 6 x 6 x 6, 100mg | |
| NI-0110-025 | 7 x 8 x 9, 250mg |
| Code | Dimension ∅×L (mm) |
Product |
| NI-0110-0255 | 4.6 x 40, 250mg | Straumann® XenoFlex Syringe |
| NI-0110-0505 | 5.6 x 45, 500mg |
Available in the following sizes
| Code | Dimension L×W×H (mm) |
Product |
| NI-0110-005 | 6 x 6 x 3, 50mg | Straumann® XenoFlex Block |
| NI-0110-010 | 6 x 6 x 6, 100mg | |
| NI-0110-025 | 7 x 8 x 9, 250mg |
| Code | Dimension ∅×L (mm) |
Product |
| NI-0110-0255 | 4.6 x 40, 250mg | Straumann® XenoFlex Syringe |
| NI-0110-0505 | 5.6 x 45, 500mg |